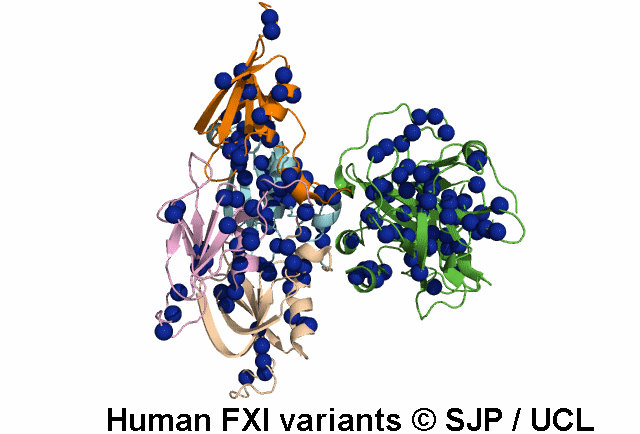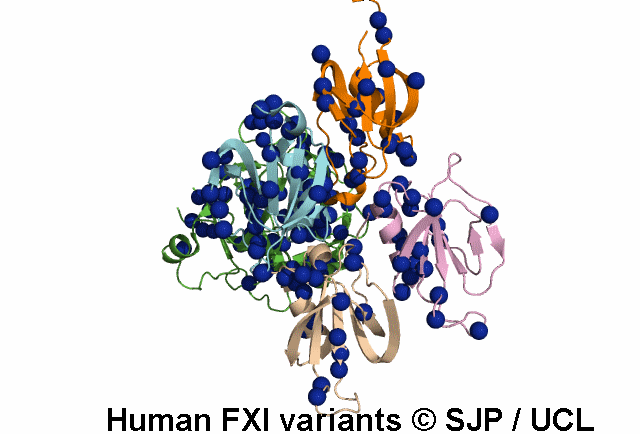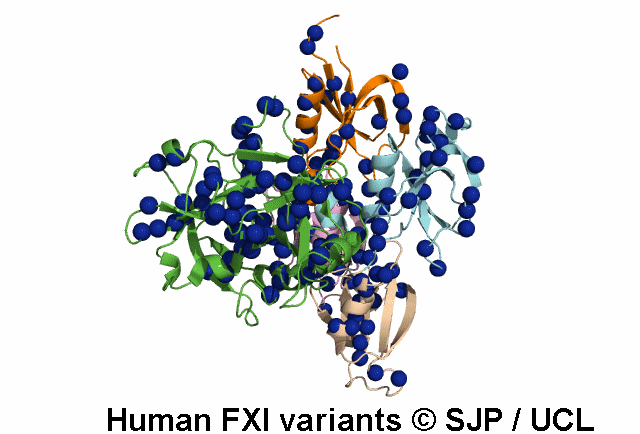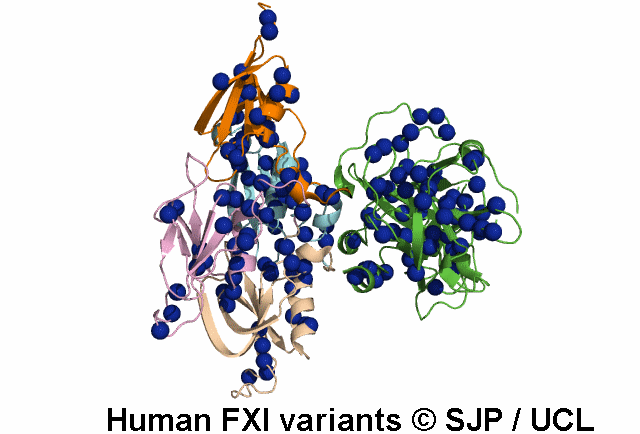
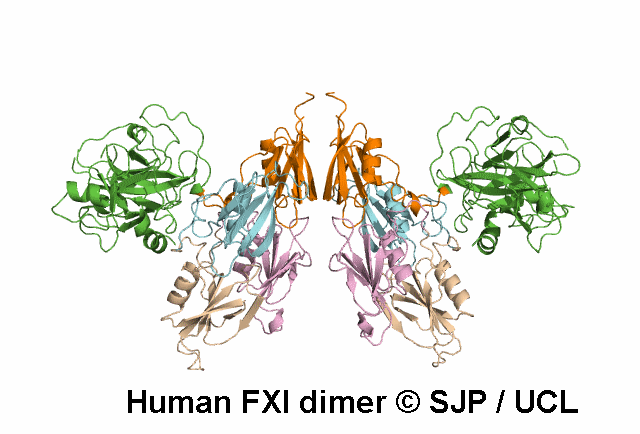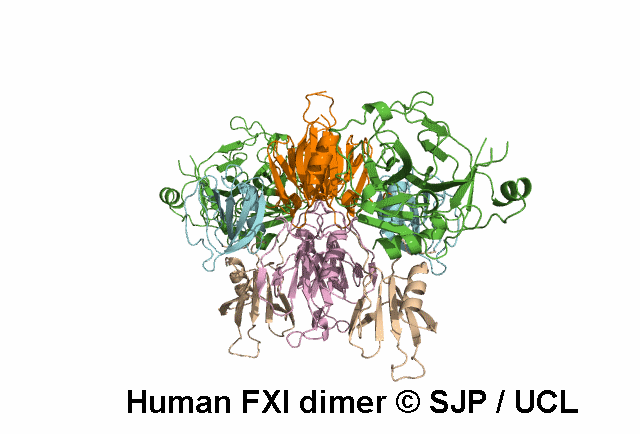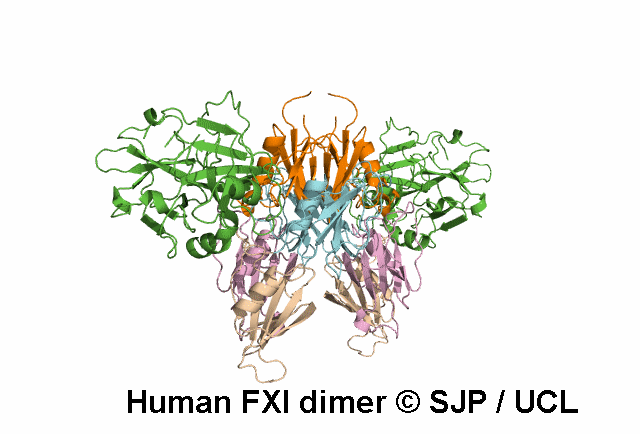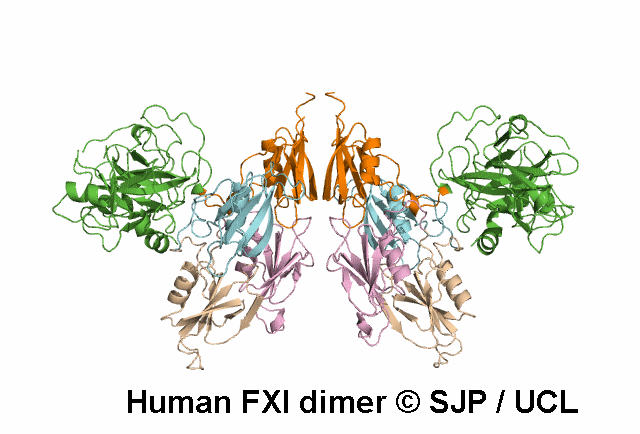In Depth Variant Analysis: c.100G>C (p.Asp34His)
Terms with a '*' next to them are explained on the Help Page .
c.100G>C
p.Asp34His (Legacy AA No. 16)
Variant Type:
Point
Domain:
Apple 1
Codon Change:
G>C
Variant Effect:
Missense
No. of Patients Reported:
0
Phenotype:
I
Allele Count *:
2
Allele Number *:
251446
Allele Frequency *:
0.000008
Variant Comments & Reference:
Amount of mutant protein secreted from cells in vitro is reduced. The replacement of Asp 34 in the Ap1 domain of factor XI is a radical substitution, because the wild-type Asp side chain is small and negatively charged at physiologic pH, whereas the His group is bulky and hydrophobic with neutral charge - the alteration of charge and shape and the resultant conformational change of this region of the molecule might interfere with chain folding. Pugh et al 1995Residue Information:
| Name | Type | Cyclic | Size | Position | Hydrophobicity | Charge | |
|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Substitution Analysis:
Structural Implications:
Asp34 is an exposed residue (surface accessibility value = 4 ).
Asp34 is in a region of secondary structure within the FXI domains.
The DSSP assignment for this residue is ... E.
Asp34 is in a region of secondary structure within the FXI domains.
The DSSP assignment for this residue is ... E.
Hint: Left click mouse | To rotate the structure
Hint: Rotate mousewheel | To zoom in/out the structure
Hint: Right click mouse | to use applet control options
|
| Spacefill | |
| Cartoon | |
| Wireframe | |
| Trace | |
| Backbone | |
| Spin | |
| Background | |
| Disulphides | |
| Domains | |
| Alternative Colouring | |
| Labels |
Right Click on the molecule's screen for more options.
NOTE: If no Java applet appears or a Java error message is shown, please click the following LINK.