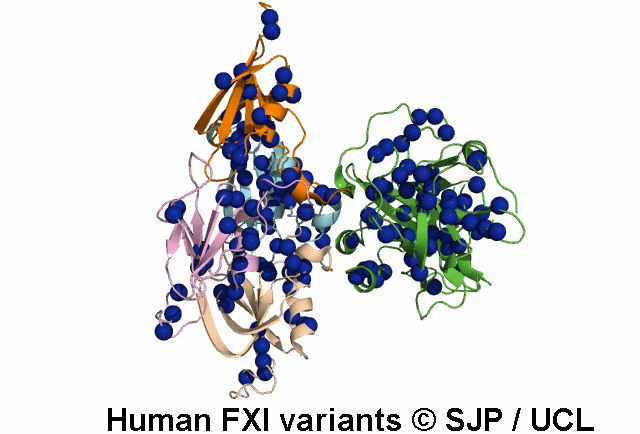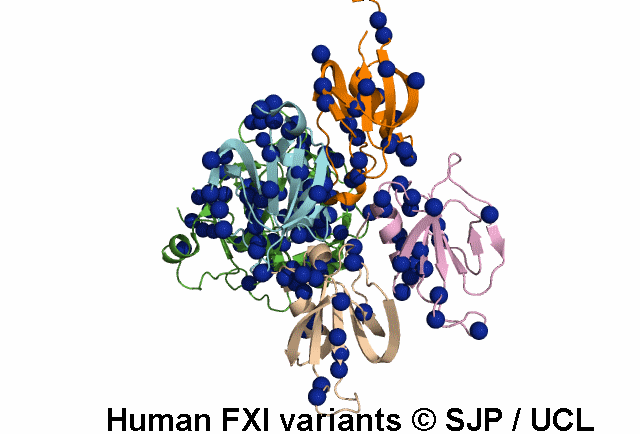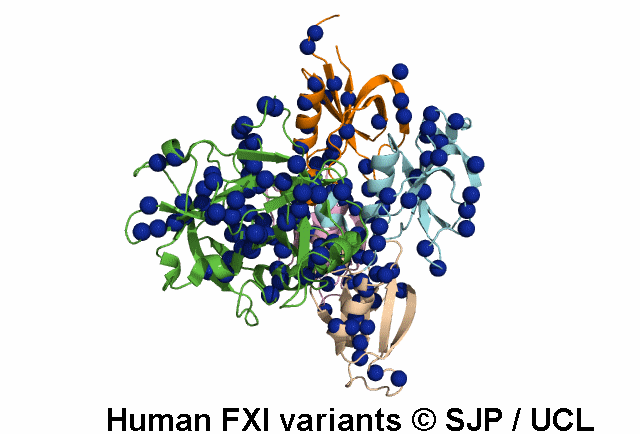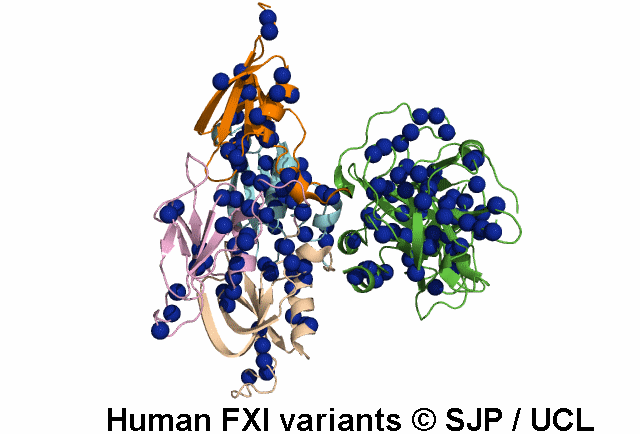
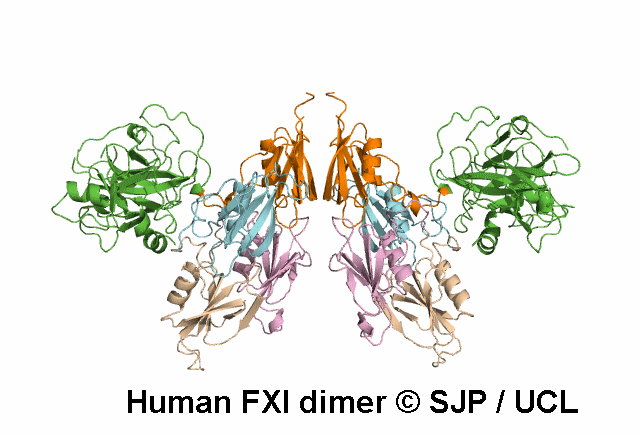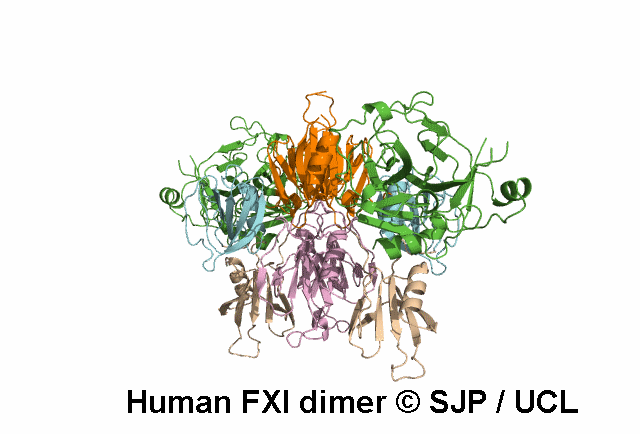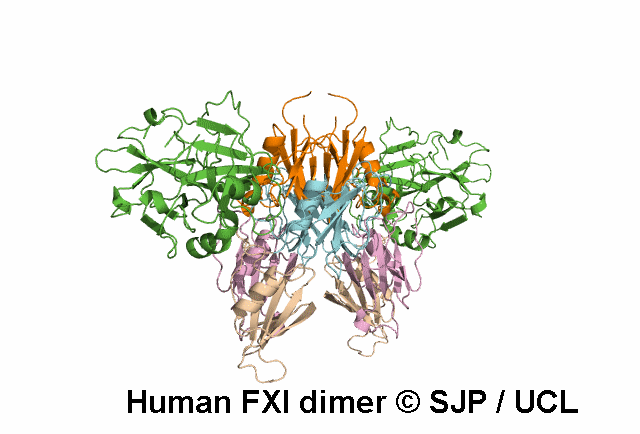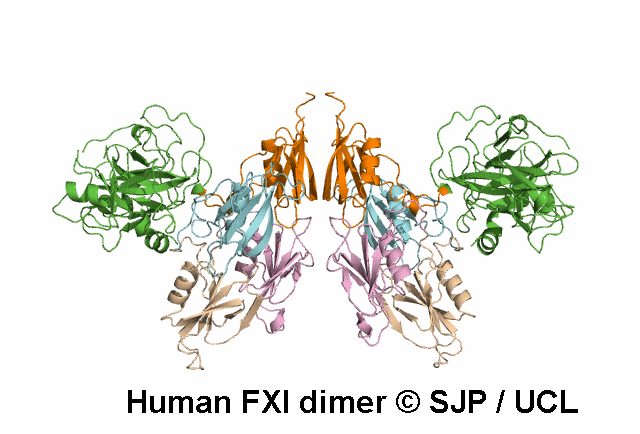FXI is a plasma glycoprotein that participates in the propagation phase of blood coagulation, augmenting thrombin generation by activating Factor IX (FIX).
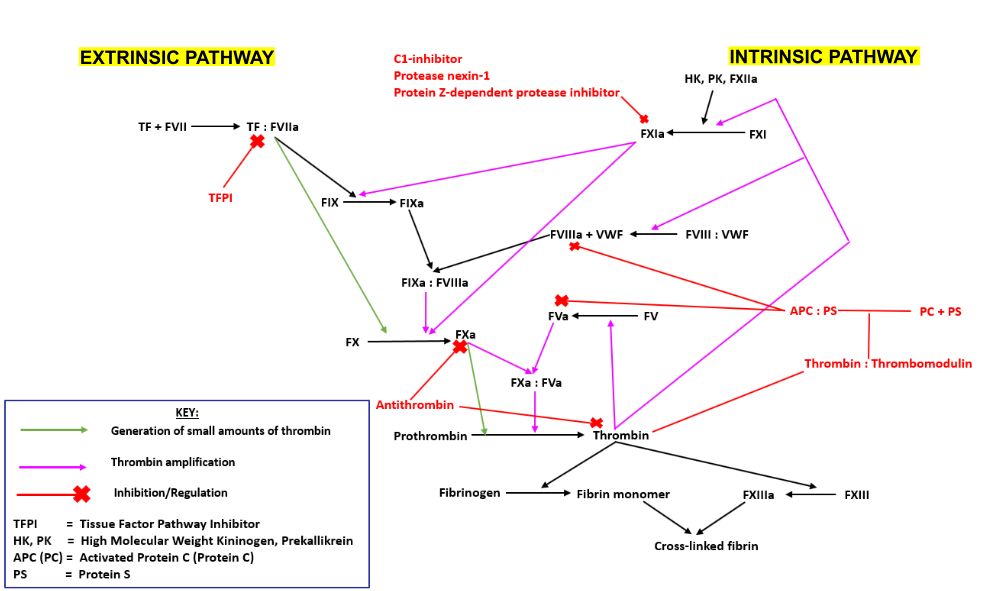
The Coagulation cascade can be divided into 3 smaller pathways;
- The extrinsic pathway is initiated following exposure of tissue factor (TF) from the vessel wall to the blood. TF forms a complex with Factor VII (FVII), which activates Factors IX (FIX) and X (FX). Activated FX converts prothrombin into thrombin. Thrombin subsequently catalyses the activation of FXI, establishing a positive feedback loop and amplifying its production.
- The intrinsic pathway is initiated upon contact of endothelial collagen with Factor XII (FXII), which itself is complexed with Prekallikrein (PK) and High Molecular Weight Kininogen (HK). Activated FXII catalyses the activation of FXI, which then interacts with the extrinsic pathway, activating FIX.
- The final common pathway involves the thrombin-mediated conversion of fibrinogen into fibrin. Thrombin activates Factor XIII (FXIII) which cross links the fibrin, enhancing its resistance to dispersion by high blood pressure.
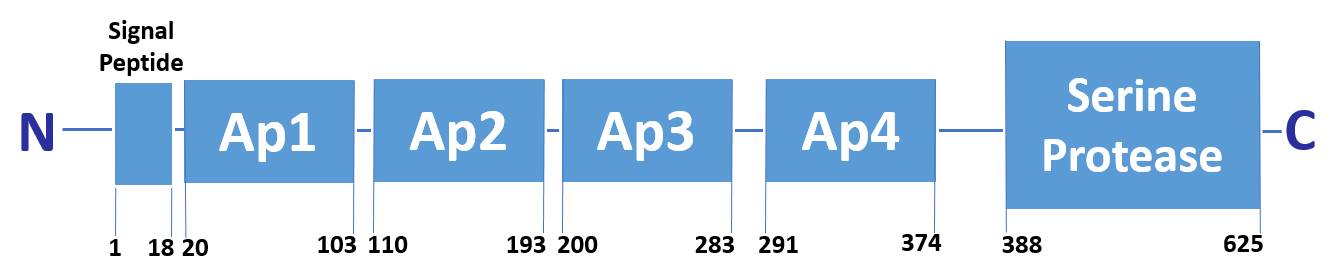
FXI is a unique coagulation protease in that it contains four tandem apple (Ap) domains , also known as PAN modules. These Ap domains show sequence homology with each other and with other Ap domains in proteins such as Hepatocyte growth factor and PK.
FXI also contains a serine protease (SP) domain that is homologous to the SP domains of other coagulation factors.
FXI circulates as a dimer, with two identical FXI monomers linked by non-covalent interactions between the Ap4 domains and by a Cys339-Cys339 (Cys321-Cys321) disulphide bond, although the disulfide bond is not essential for dimerisation.
FXI can be activated by thrombin or by activated FXII. Alternatively, FXII may undergo auto-activation. Activation of FXI results in cleavage of the scissile bond at Arg387-Ile388 (Arg369-Ile370), forming a heavy chain containing the Ap domains and a light chain containing the SP domain. The SP domain contains the catalytic triad which lies at His431 (His413), Asp480 (Asp462) and Ser575 (Ser557).
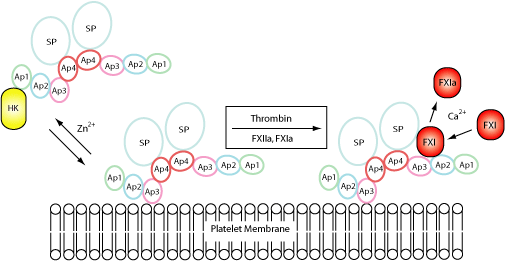
FXI is activated when bound to activated platelets. The FXI Ap3 domain binds to the platelet glycoprotein (GP) Ib-IX-V complex in the presence of HK and zinc ions, or prothrombin and calcium ions. Binding sites for HK and PK have been mapped to the Ap1 domain and a binding site for FXIIa has also been proposed within the Ap4 domain. The Ap2 and Ap3 domains both seem to be involved in binding FIX, which is cleaved by the SP domain.
Variants within the F11 gene can cause FXI deficiency, also known as Haemophilia C, Plasma thromboplastin antecedent deficiency and Rosenthal syndrome. Individuals with FXI deficiency display variable clinical phenotypes.
FXI coagulant activity (FXI:C) in normal human plasma is 70-150 IU/dL. Severe FXI deficient patients have FXI:C levels of < 1-20 IU/dL , whereas partially
deficient FXI deficient patients possess FXI:C levels of 20-70 IU/dL.
Individuals with severe FXI deficiency are homozygous or compound heterozygous for causative
mutations, whereas individuals who are partially deficient are heterozygous, with one mutated allele.
FXI deficiency is associated with a variable injury-related bleeding diathesis with bleeding symptoms associated with trauma or surgery and a lack of spontaneous bleeding.
The majority of severely deficient individuals bleed excessively after invasive procedures but this is not universal. Up to 50% of partially deficient patients have a history of bleeding.
In females, menorrhagia (heavy or prolonged bleeding during menstruation) and post-partum hemorrhage (bleeding after pregnancy) are particular features.
The bleeding tendency varies considerably between patients with similar FXI levels and may also vary within the same individual.
Bleeding is more common after surgical procedures on areas of high fibrinolytic activity such as the oral and nasal cavities, prostate and uterus.
Current available therapy for FXI deficiency consists of FXI replacement and the use of antifibrinolytic agents.
- FXI replacement is achieved using fresh frozen plasma or FXI concentrate. While FXI replacement is effective in the prevention of surgical bleeding, lack of availability or contra-indications to fresh frozen plasma or FXI concentrate may preclude FXI replacement in a significant number of patients.
- Antifibrinolytic agents are often used as bleeding in FXI deficient individuals is typically associated with areas of high fibrinolytic activity. An example of an antifibrinolytic agent is tranexamic acid, which, due to ease of administration (can be given in tablet or mouthwash form), is one of the most frequently used agents. Tranexamic acid is particularly effective in preventing bleeding after dental extractions.
- Recombinant factor VIIa has been used successfully in the prevention of surgical bleeding in a small number of FXI deficient patients.